Cleanroom Solutions
From over two decades of collaborating with businesses of all sizes and sectors, our cleanroom solutions have been built by you, for you. With trusted designs, proven expertise, and everything you need from one reliable source, we take the complexity out of the cleanroom ordering process.
Simply:
- Choose a design
- Place your order
- Receive your solution
So, whether you’re introducing a new cleanroom or expanding an existing facility, our predesigned solutions will get you set up and running in no time.

Our cleanroom range
Predesigned and prefabricated cleanrooms and laminar flow cabinets, all designed to popular sizes and requested specifications, taking the complexity out of the cleanroom ordering process.
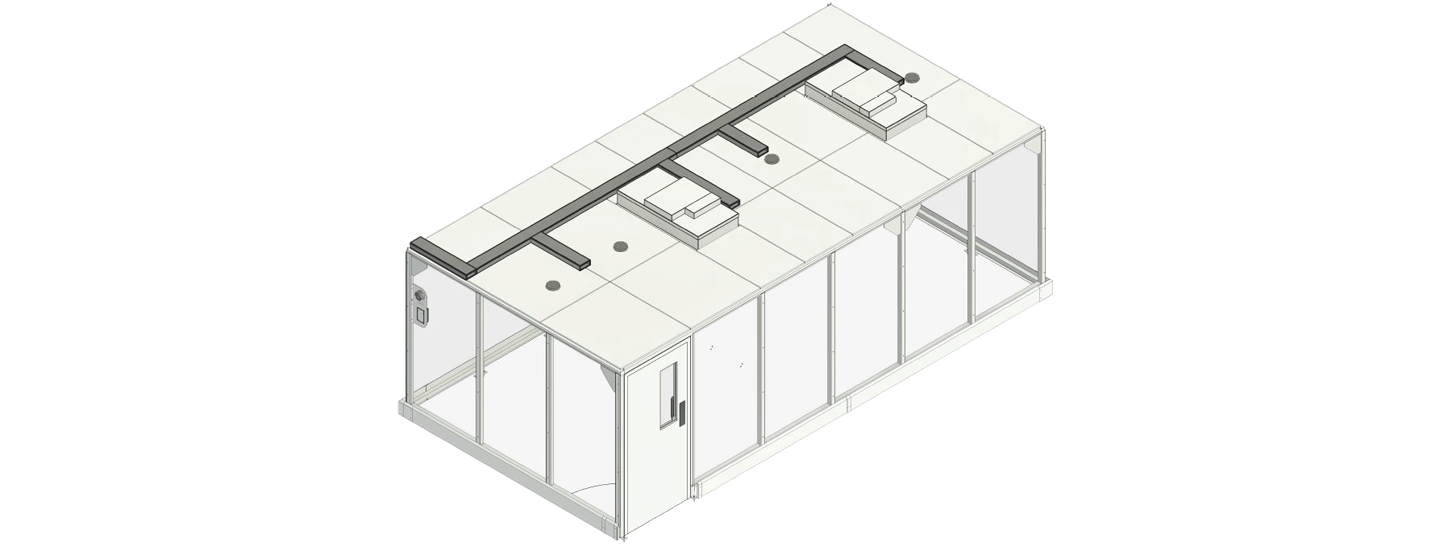
Predesigned cleanrooms
Built with a powder-coated steel frame and a choice of wall panels, these high-integrity modular cleanrooms are a resilient, future-proof solution. Whether you need a portable cleanroom or one with an integrated change area, we have you covered!

Laminar flow cabinets
Create an ISO class 5 working environment within an enclosed tabletop space, these laminar flow cabinets are designed to seamlessly integrate into your existing facility, for processes that require a tighter level of contamination control.
Why choose one of our cleanroom solutions?
Our prefabricated cleanrooms and laminar flow cabinets have been carefully developed over several years, taking the time and consideration to ensure they meet the popular size and specification requirements our customers look for.
With features that just make sense, such as variable fan speed control, LED lighting and HEPA fan filtration, our contamination control solutions meet the cleanliness standards and functionality commonly requested from a wide range of sectors, including injection moulding, R&D, manufacturing, aerospace, education, start-up’s and more.
Effective design
Speedy delivery
Easy installation
Assisted application
You'll be in good company
Setting up your new cleanroom just got easier
Complementary cleanroom starter packs
Not only were we the first ecommerce provider of cleanroom consumables and furniture, we’ve also been designing, building, and maintaining cleanrooms for over 20 years. With over two decades of working closely with our clients, we’ve learnt what it takes to effectively and consistently create and maintain an optimal controlled environment.
To help make setting up your new cleanroom even easier, we’re now offering a complementary Cleanroomshop starter pack filled with essential cleanroom items. From cleanroom clothing, disinfectants, and contamination control solutions, our specialists have hand-picked the most popular and sought-after items. Plus, you’ll also receive a £50 voucher and exclusive discounts to spend on Cleanroomshop for your next order!
When you partner with us for your next project, we’ll do more than just build your cleanroom; we’ll provide you with the tools and essentials to optimise your production process.

Frequently asked questions
We offer a range of cleanroom solutions including predesigned hardwall and softwall modular cleanrooms, and laminar flow cabinets. The right option for you will depend on your process, by our cleanroom experts will support you with choosing the most appropriate option for your sector and application.
Our cleanroom solutions vary is cost depending on type and tailored additions; however, our laminar flow cabinets start from just £3,995 +VAT and our predesigned cleanrooms start from just £5,095 +VAT, offering an extremely cost-effective controlled environment.
Yes. It is recommended to validate your cleanroom solution every 12 months; however, validation and any cleaning regimes should be based on your own risk assessments.
Ultimately, this depends on your own process and risk assessment. If you aren’t sure, speak with your regulatory body and auditors to find out what their expectations are. You can also speak with a member of our team who will support you with selecting the right cleanroom solution for your business.
We aim to deliver your new cleanroom solution within 5 weeks of your purchase date. However, this is dependent on stock availability and delivery location, which in some cases may extend lead times.


Start a project with us
Our design and build specialists have experience working with customers in all kinds of industries on a global scale, achieving great results time and time again. We’d love to work with you as well!




