Predesigned Cleanrooms
Our range of predesigned cleanrooms are a swift, convenient, and cost-effective contamination control solution for entry-level facilities or a quick way to react to growing production environments.
Predesigned by our design and build experts, and constructed in as little as 48 hours by our engineers, choose from popular sizes and specifications suited to a wide range of applications and industries.
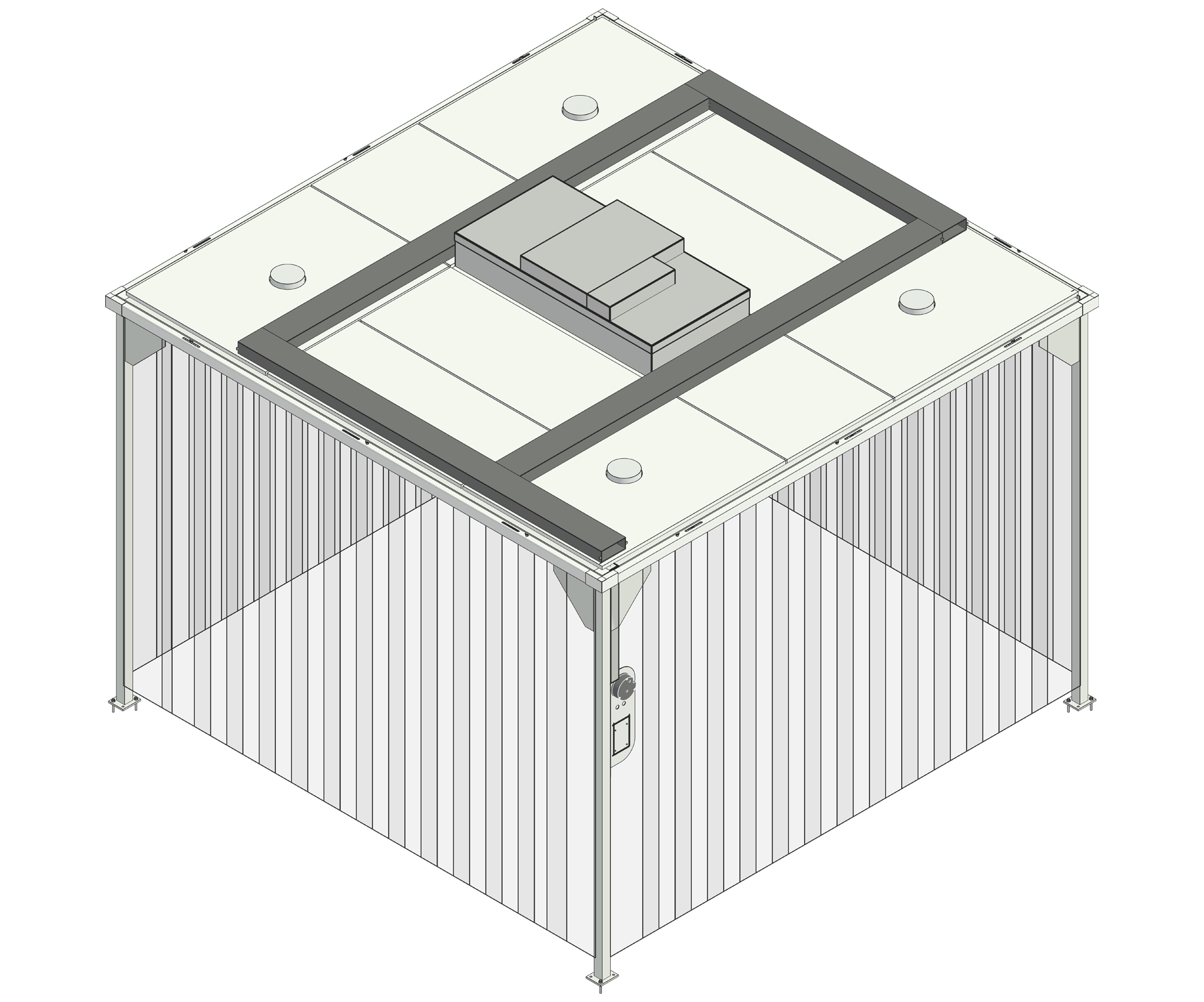
What are predesigned cleanrooms?
With over 20 years’ experience designing, building, and validating cleanrooms worldwide, we’ve placed our expertise into developing a predesigned cleanroom range.
Choosing a predesigned cleanroom means you skip the design phase associated with custom-built projects, reducing lead times and keeping costs down.
Built to a predetermined design and utilising standard components, each cleanroom can be easily dismantled and reconstructed, achieve an ISO class 7 operating environment, and be installed within just 48 hours.

So, whether you’re a start-up business, or wanting to expand your existing capacity, these predesigned cleanrooms are ideal for those who need a cost-effective, quick solution that works within their budgets and time frames.
Ready to start your next project? Contact us below!

Explore the predesigned cleanroom range
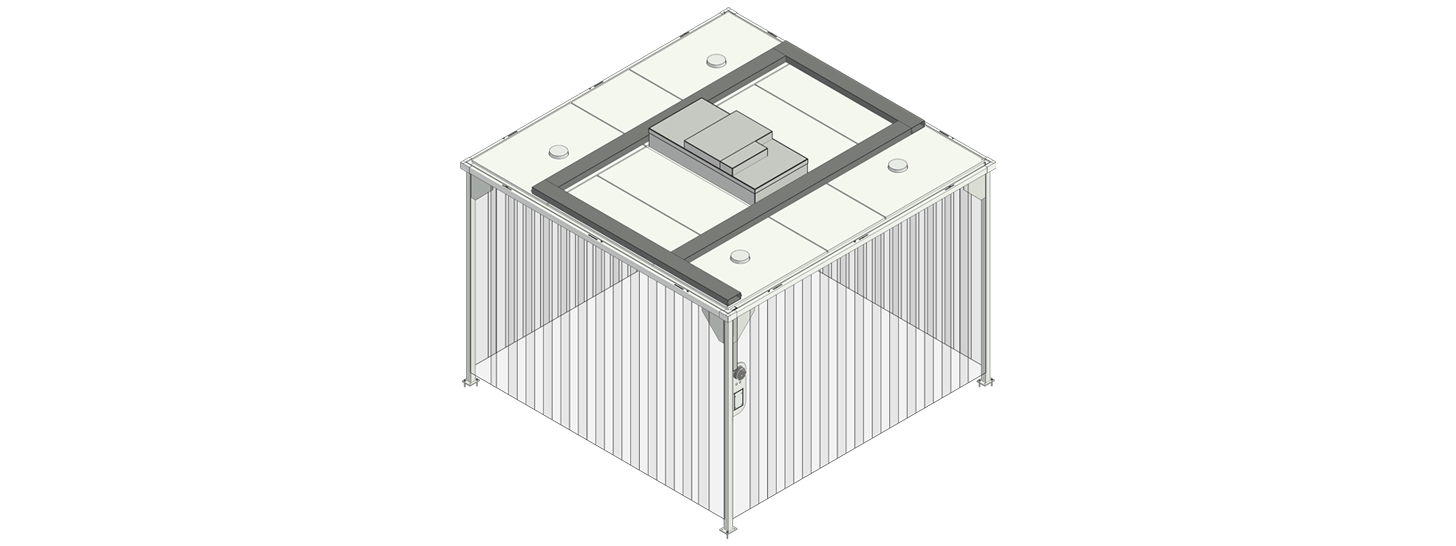
Predesigned hardwall cleanrooms
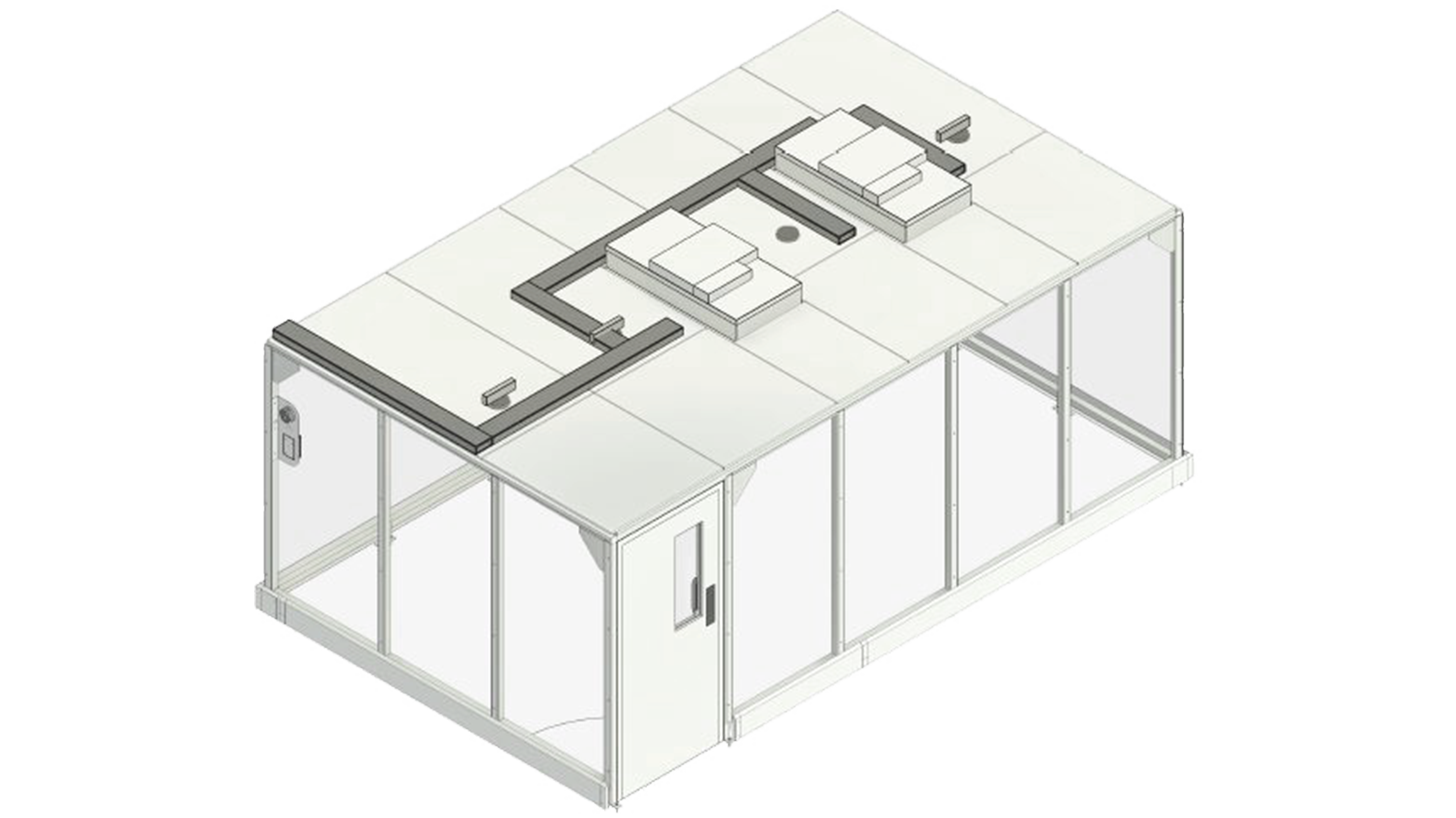
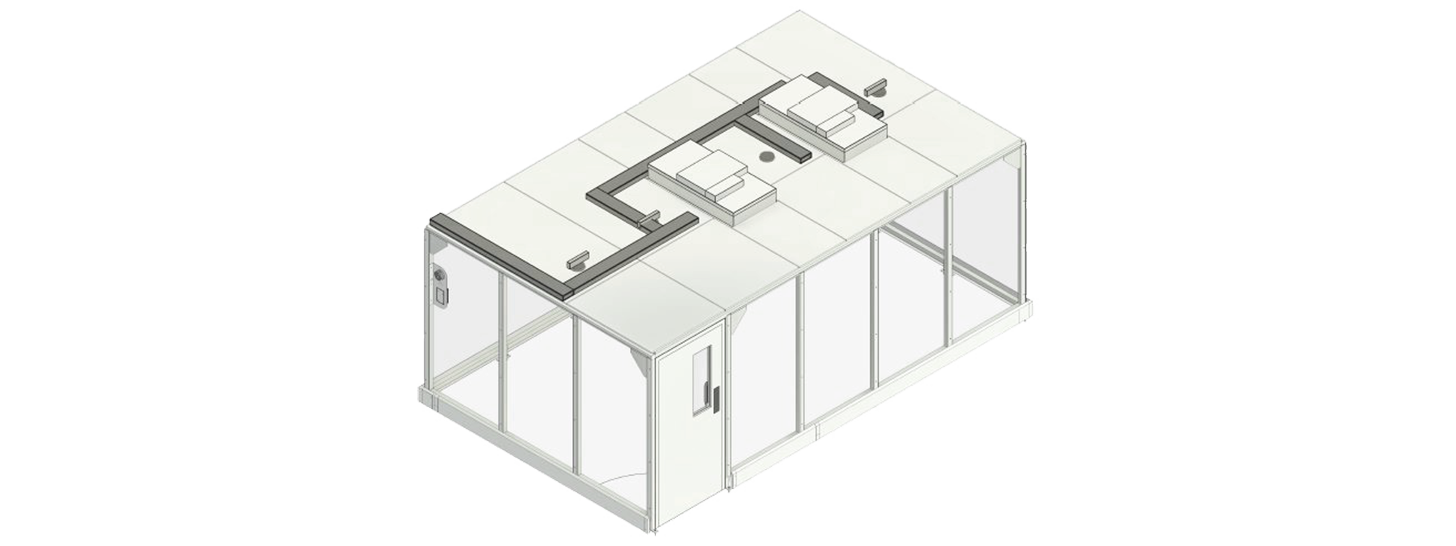
Predesigned softwall cleanrooms
The predesigned cleanroom process
With a predesigned cleanroom, you can get set-up and ready to start your production process in just three, simple steps:
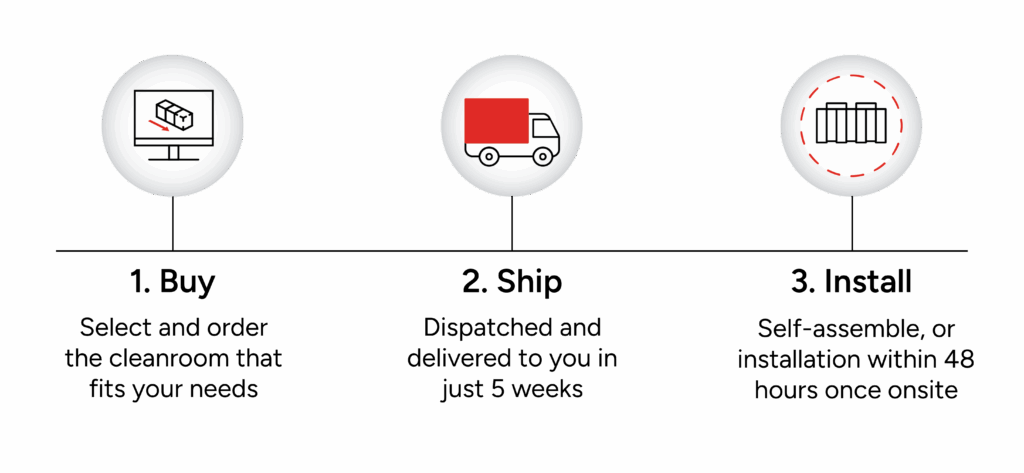
Why choose a predesigned cleanroom?
Reduced lead times
Speedy construction time
Cost effective
Change area
ISO class 7
Popular sizes
Choosing the right cleanroom for your operations
| Predesigned hardwall cleanrooms | Predesigned softwall cleanrooms | |
|---|---|---|
| 5-week lead time | ✔ | ✔ |
| 48-hour installation | ✔ | ✔ |
| Flooring* | ✔ | ✔ |
| Castors | ✘ | ✔ |
| Door | ✔ | ✘ |
| LED lighting | ✔ | ✔ |
| Change area | ✔ | ✘ |
| Pressue gauge | ✔ | ✔ |
| Speed control | ✔ | ✔ |
| Internal walls | ✔ | ✘ |
| External walls | ✔ | ✔ |
| Trunking** | ✔ | ✘ |
| Transfer hatch** | ✔ | ✘ |
*Optional addition to predesigned hardwall and softwall builds
**Optional additions to predesigned hardwall builds
Looking for something a little more bespoke?
Connect 2 Cleanrooms offer a complete cleanroom design and build service. Our bespoke modular cleanrooms create classified environments to protect processes from harmful airborne contamination – reducing failure rates and helping organisations get it right first time.
We manufacture a cleanroom of a few square meters, to hundreds of square meters, with the ability to install bespoke designs around existing machinery where needed. The modular design means that our cleanrooms can be extended or relocated, so you can grow your production areas as demand increases.
Ready to start a new project? Request a quote below!

Frequently asked questions
Our predesigned cleanroom range is designed to meet ISO 14644-1 class 7 requirements. If you’re looking for a higher classification – such as ISO class 6 for example – we can modify and customise the range to achieve these standards.
Yes! Our predesigned cleanroom range can be customised to suite your specific needs and requirements – for more information, speak to one of our experts who’ll be happy to support with your next project.
Our predesigned cleanroom range can be delivered within 5 weeks and installed within 48 hours of our engineers arriving on site.
Yes! Our predesigned cleanroom range has been developed to be used either as a standalone cleanroom, a bolt-on to an existing controlled environment, or with the intention to expand later down the line.
Like custom-built cleanrooms, predesigned cleanrooms require a validation and maintenance process in line with ISO 14644-1 standards, including a validation every 12 months and regular cleaning schedule.
The softwall cleanrooms have the option to come with castors, and hardwall cleanrooms can be easily deconstructed and reconstructed.
Our predesigned cleanroom range is perfectly suited to a wide range of sectors and applications.




